
SF process, either isoergic or slightly exoergic in producing two triplet excitons with a net spin of zero, is spin-allowed and favorable for fast generation of doubled triplet excitons from high-lying singlet excitons, when S 1 excitation energy is comparable with twice the energy of T 1 excitation ( E T1/ E S1 < 0.5) 18.Įxtensive efforts have been so far devoted to reducing Δ E ST via separated the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) strategy to construct efficient TADF molecules 8.
Meanwhile, when the Δ E ST is increased and the energy of two triplet excitons are close to, or larger than, one singlet exciton ( E T1/ E S1 ≳ 0.5), TTA could happen between triplet exciton interaction pair following the spin statistics rule 17. 1, when Δ E ST normally laid between 0.5 and 1.0 eV 7 in conventional compounds is reduced (Δ E ST ≤ 0.37 eV) 15, TADF could be resulted via activated endothermic RISC process from T 1 to S 1 by the thermal motions of the molecule atoms for the E-type delayed fluorescence 16. To control the triplet/singlet excited states in a designed manner for a desired optoelectronic property, the rational adjustment of the singlet-triplet energy gap (Δ E ST) between the first singlet (S 1) and triplet (T 1) excited states is the key. Notably, the TTA compounds, which can harvest one singlet exciton from two low-lying triplet excitons, can benefit OLEDs with improved external quantum efficiency (EQE) theoretically up to 12.5% by harvesting the 75% electronically generated triplet excitons to produce singlet excitons for fluorescence 9 the SF process, which transforms a singlet exciton into two triplet excitons on neighboring molecules with EQE up to 200%, is especially attractive for solar cells in providing doubled photocurrent from high-energy photons 10 the recently developed TADF materials by harvesting 100% triplet excitons via reversed intersystem crossing have achieved EQEs of 20.6% in blue and 30.0% in green TADF OLED devices 11, 12, which are comparable to the heavy metal-based phosphorescent emitters 13, 14. The rich photophysical properties of organic molecules have led to many revolutionary developments in organic electronics 8. The ultimate challenge in manipulating conjugated molecules 1, 2 for optoelectronic applications is to develop universal approaches capable of controlling excited states for efficient electron-light conversions, affording not only conventional fluorescence 3 and phosphorescence 4, but also many other photophysical phenomena including triplet-triplet annihilation (TTA) 5, singlet fission (SF) 6 and thermally activated delayed fluorescence (TADF) 7. These findings provide keen insights into Δ E ST control for feasible excited state tuning, offering valuable guidelines for the construction of molecules with desired optoelectronic properties. Importantly, we realized a widely-tuned Δ E ST in a range from ultralow (0.0003 eV) to extra-large (1.47 eV) via a subtle symmetric control of triazine molecules, based on time-dependent density functional theory calculations combined with experimental explorations. These critical parameters revealed that both separated S 1 and T 1 states leads to ultralow Δ E ST separated S 1 and overlapped T 1 states results in small Δ E ST and both overlapped S 1 and T 1 states induces large Δ E ST. Here, we demonstrate a convenient and quantitative approach to relate Δ E ST to the frontier orbital overlap and separation distance via a set of newly developed parameters using natural transition orbital analysis to consider whole pictures of electron transitions for both the lowest singlet (S 1) and triplet (T 1) excited states. The variation of singlet-triplet splitting (Δ E ST) can provide useful means in modulating organic excitons for diversified photophysical phenomena, but controlling Δ E ST in a desired manner within a large tuning scope remains a daunting challenge.
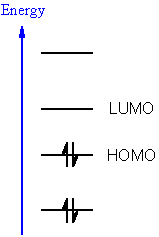
Developing organic optoelectronic materials with desired photophysical properties has always been at the forefront of organic electronics.


 0 kommentar(er)
0 kommentar(er)
